Scientists Developed Material Enabling Chemical Production Using Sunlight and Water
Hydrogenation reactions play a key role in organic chemistry, the pharmaceutical industry, and agrochemistry. These processes involve the conversion of organic compounds using gaseous hydrogen and typically require high temperatures and pressures. As a result, they are both economically costly and environmentally challenging due to high energy consumption and carbon emissions. An international team of researchers from the Czech Republic, Germany, and China has developed a novel material using nanotechnology and atomic engineering that can convert a wide range of organic compounds into desired products under ambient conditions, using only sunlight and water as a proton source. Scientists from the Center for Energy and Environmental Technologies (CEET) at VSB – Technical University of Ostrava and Czech Advanced Technology and Research Institute (CATRIN) at Palacký University Olomouc played a key role in designing the material and uncovering its reaction mechanism. The findings were published in the prestigious journal Advanced Materials.
Hydrogenation reactions are integral to hundreds of chemical processes across agrochemistry, pharmaceuticals, and industrial chemistry, representing a global market worth tens of billions of dollars. Finding new chemical pathways for reducing organic compounds under mild conditions—without the use of gaseous hydrogen—is a major scientific and industrial challenge. One promising approach involves using water as a proton source and effective photocatalysts to drive reactions with solar energy, eliminating the need for hydrogen gas and energy-intensive conditions.
“Unfortunately, existing photocatalysts do not achieve the yields required for industrial-scale applications and suffer from limited selectivity, meaning they struggle to direct reactions toward specific desired products. Moreover, they often require co-reagents, such as magnesium to activate water, and are only applicable to a narrow range of organic reactions,” explained Radek Zbořil, a leading scientist working at CATRIN and CEET.
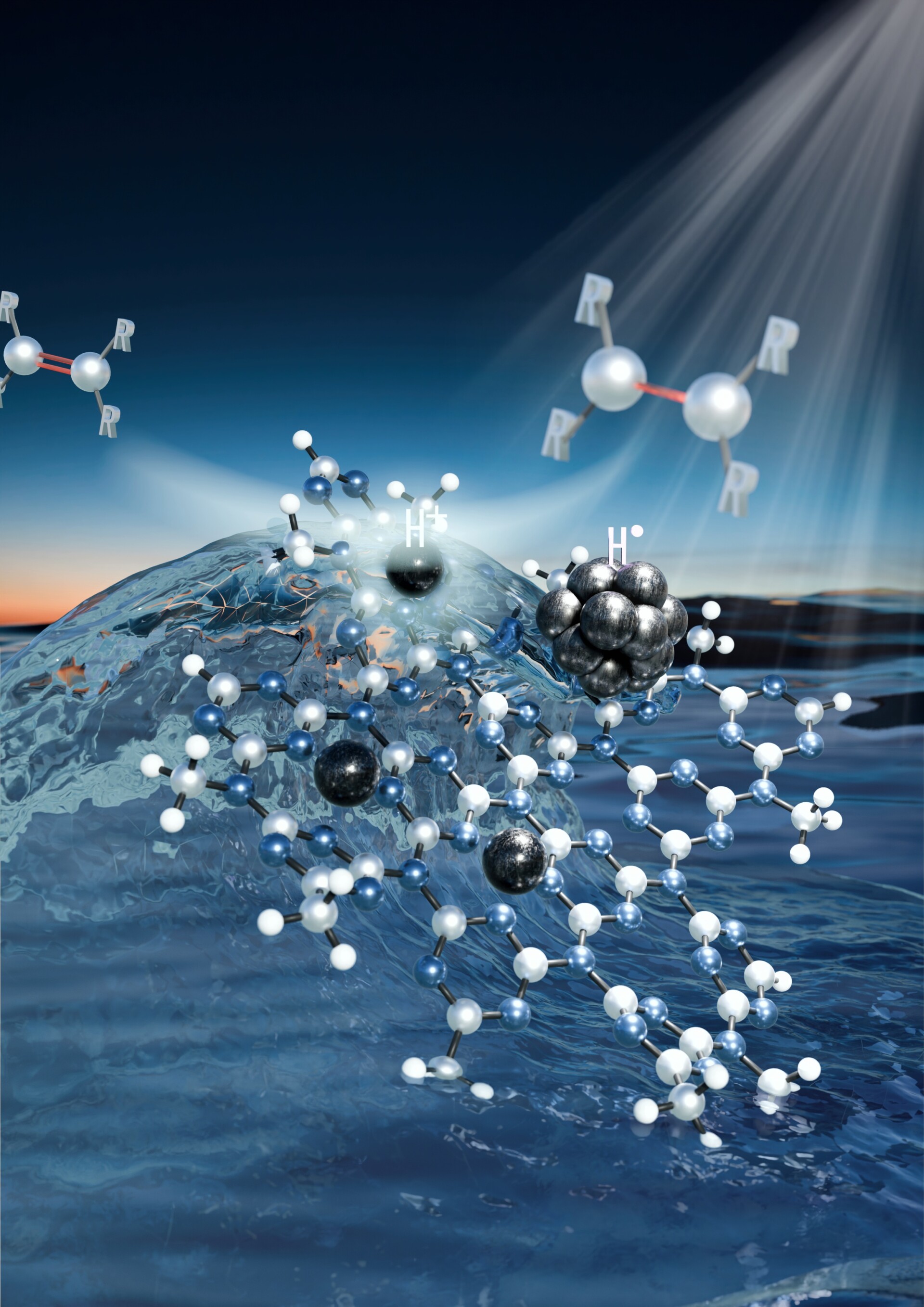
“In developing a new type of photocatalyst, we decided to combine our expertise in nanotechnology and atomic engineering. Together with our international collaborators, we designed a material comprising palladium nanoparticles anchored within a two-dimensional carbon nitride matrix, with isolated palladium atoms in various oxidation states embedded nearby. Thanks to the synergistic effect of all components, this new material achieved exceptionally high yields and selectivity in converting a broad spectrum of organic compounds—opening the door to industrial applications,” Zbořil further stated.
Initially, the researchers examined the use of palladium nanoparticles alone within the carbon nitride structure, as well as the effectiveness of isolated palladium atoms in the same photocatalytic matrix. Both approaches yielded relatively low efficiencies—until a serendipitous observation pointed them in a new direction.
“We noticed that when palladium atoms in various oxidation states were located very close to the nanoparticles, reaction yields increased dramatically,” said Giorgio Zoppellaro, also affiliated with CATRIN and CEET, who played a key role in elucidating the material’s mechanism of action. “We then deliberately designed a composite system in which the isolated palladium atoms attract photo-generated holes for water oxidation, while the nanoparticles facilitate hydrogen transfer to unsaturated bonds in organic molecules. This represents a unique synergy between nanotechnology and atomic engineering that could transform catalytic processes in organic chemistry, with huge potential in fields like pharmaceuticals and agrochemicals,” Zoppellaro concluded. Research teams at CATRIN and CEET, led by Prof. Zbořil, focus on hydrogenation reactions in collaboration with colleagues from the Leibniz Institute for Catalysis (LIKAT) in Germany for many years. They have previously published several groundbreaking studies, particularly on new technologies for producing amine compounds via hydrogenation (e.g., Chandrashekhar et al. Nature Catal. 2022; Cheruvathoor Poulose et al. Nature Nanotech. 2022).